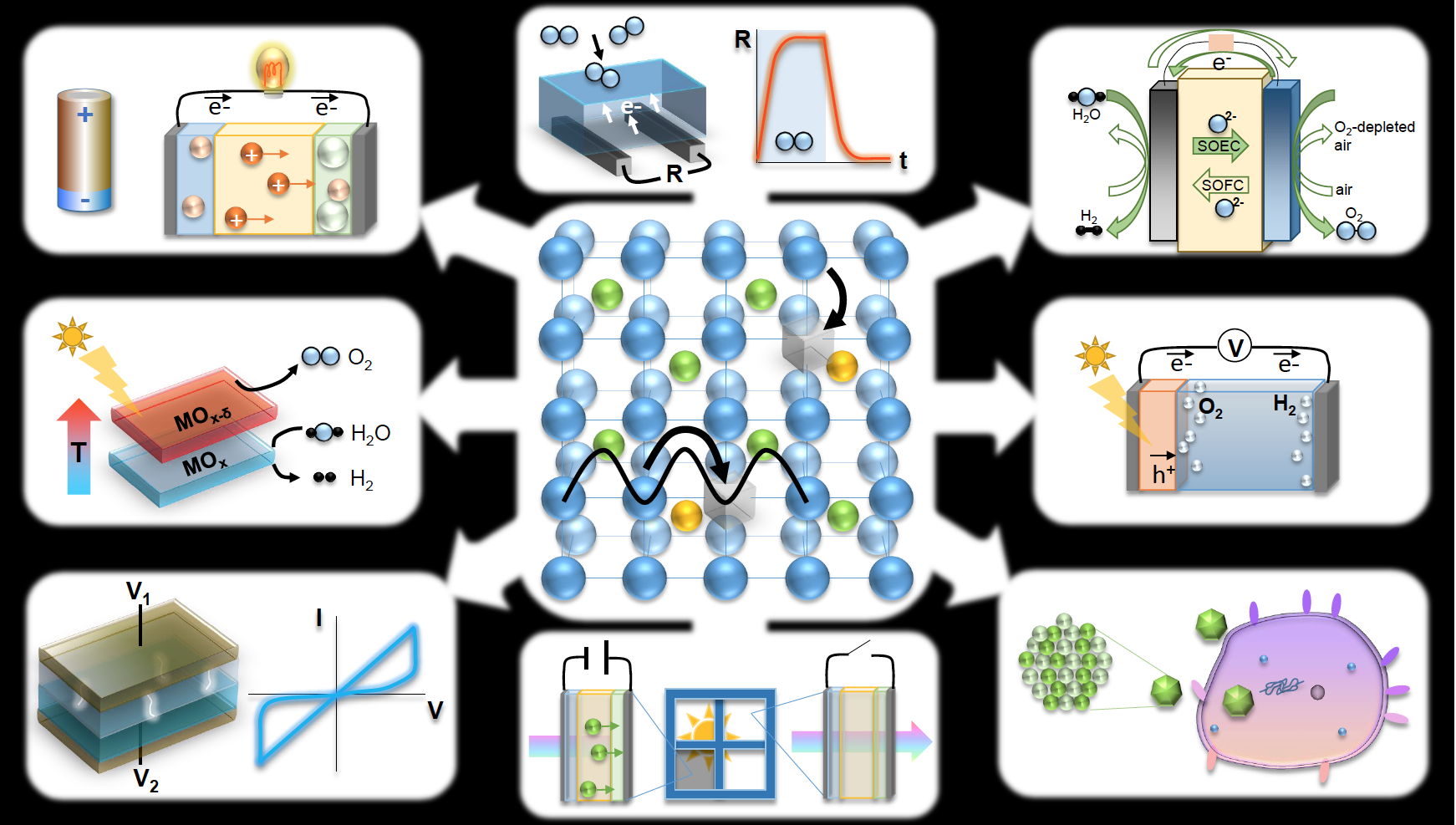
Courtesy of Dr. Nicola Perry, Kyushu University
From top left, clockwise:
1) BATTERY: An ion chemical potential difference across the cell electrolyte enables electrical work to be done on an external load (discharging) or energy storage (charging) at room temperature (energy conversion/storage)
2) CHEMI-RESISTIVE SENSOR: Gas adsorption by electron-withdrawing species increases the measured n-type resistance in sensor material with high surface-to-volume ratio, enabling gas sensing (environmental monitoring)
3) SOLID OXIDE CELL: High temperature, clean, and efficient electrochemical power generation from various fuels, or chemical fuel production via electrolysis within the same reversible device (energy conversion/storage)
4) PHOTOELECTROCHEMICAL CELL: Electrons and holes, generated by incident sunlight in semiconductor, separate under internal electric field and drive the water splitting reaction generating fuel and oxygen (energy storage)
5) BIO-IONICS: Interactions between biological cells and redox active nanoparticles have implications for toxicity and antimicrobial applications; additionally, biological ion transport suggests possibilities for biomimetic solid state ionics (health)
6) PHOTO-CHROMICS: reversible voltage-driven ion motion changes optical absorption of window, giving control of light transmission with applications to smart windows and displays (energy conservation)
7) MEMRISTOR/ RESISTIVE SWITCH: voltage-driven changes in resistance state owing to formation of conductive regions or filaments enable new applications in memory and computing (nanoelectronics)
8) SOLAR THERMOCHEMICAL CELL: solar heating causes mixed conductor to lose oxygen; upon cooling materials are thermodynamically driven to pick up oxygen, for example turning water into useful fuel (energy storage)
