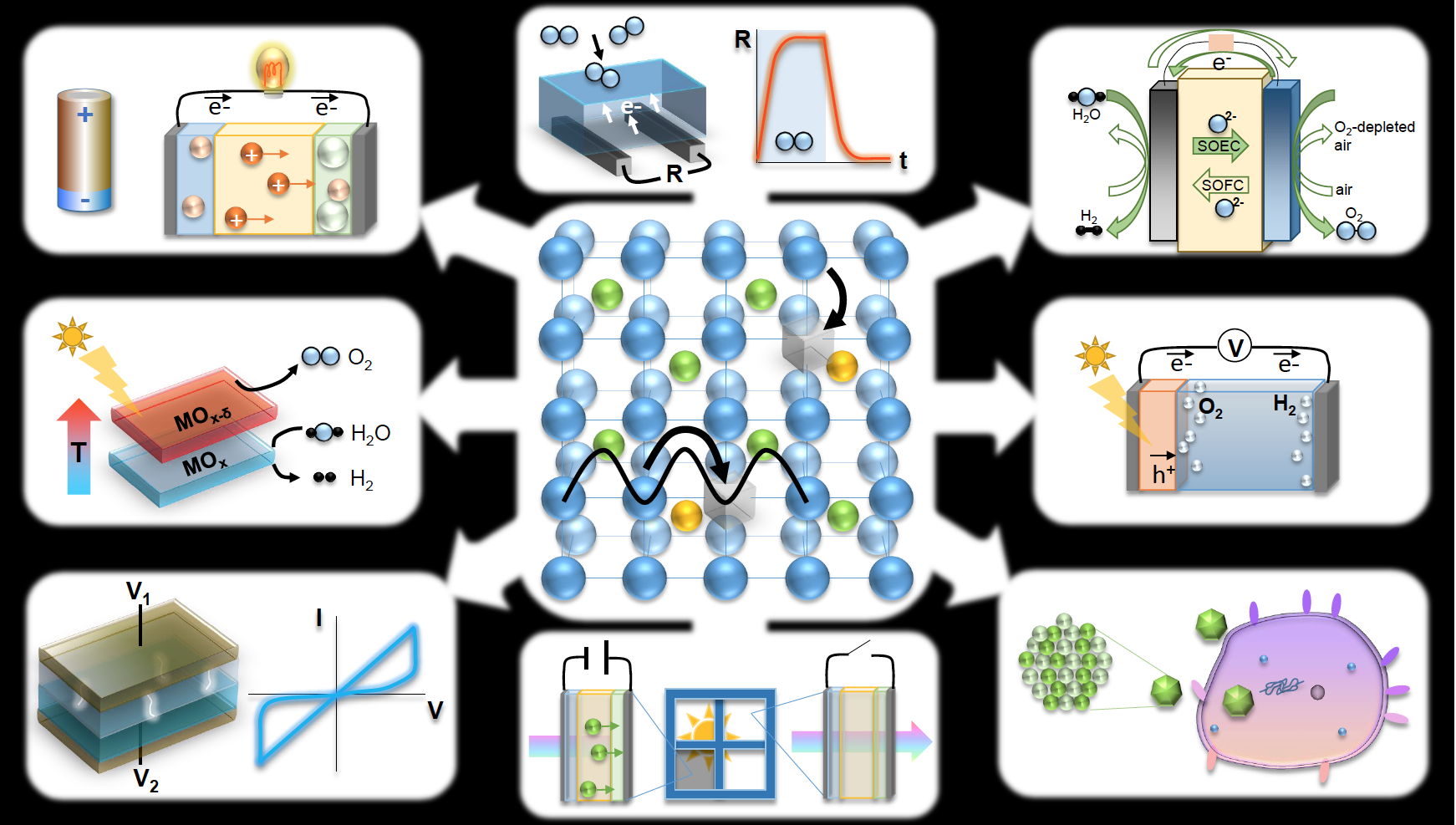
Courtesy of Dr. Nicola Perry, Kyushu University
From top left, clockwise:
1) BATTERY: An ion chemical potential difference across the cell electrolyte enables electrical work to be done on an external load (discharging) or energy storage (charging) at room temperature (energy conversion/storage)
2) CHEMI-RESISTIVE SENSOR: Gas adsorption by electron-withdrawing species increases the measured n-type resistance in sensor material with high surface-to-volume ratio, enabling gas sensing (environmental monitoring)
3) SOLID OXIDE CELL: High temperature, clean, and efficient electrochemical power generation from various fuels, or chemical fuel production via electrolysis within the same reversible device (energy conversion/storage)
4) PHOTOELECTROCHEMICAL CELL: Electrons and holes, generated by incident sunlight in semiconductor, separate under internal electric field and drive the water splitting reaction generating fuel and oxygen (energy storage)
5) BIO-IONICS: Interactions between biological cells and redox active nanoparticles have implications for toxicity and antimicrobial applications; additionally, biological ion transport suggests possibilities for biomimetic solid state ionics (health)
6) PHOTO-CHROMICS: reversible voltage-driven ion motion changes optical absorption of window, giving control of light transmission with applications to smart windows and displays (energy conservation)
7) MEMRISTOR/ RESISTIVE SWITCH: voltage-driven changes in resistance state owing to formation of conductive regions or filaments enable new applications in memory and computing (nanoelectronics)
8) SOLAR THERMOCHEMICAL CELL: solar heating causes mixed conductor to lose oxygen; upon cooling materials are thermodynamically driven to pick up oxygen, for example turning water into useful fuel (energy storage)
ISSI Board statement on the war against Ukraine:
The board of the International Society for Solid-State Ionics (ISSI) expresses our shock and condemnation of Russia's violent attack on Ukraine, the bloodshed, and the crises this war inflicts on its people and the world. Our thoughts are with students and researchers in Ukraine in your daily struggle to survive and work. Your suffering causes us to reflect on violence also in other parts of the world. As a very small token of our support, we waive the registration fee for any Ukrainian participant, including foreign nationals studying in Ukraine, that would be able to attend the upcoming SSI-23 in Boston this summer 2022.

